Global and European Intraosseous Devices Market Outlook 2025–2035
Surging trauma and emergency care needs drive a global boost in intraosseous devices, with APAC and GCC markets accelerating rapidly.
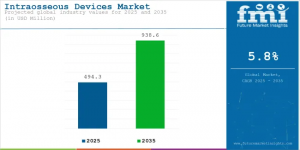
SPAIN, November 13, 2025 /EINPresswire.com/ -- The global Intraosseous Devices Market is entering a high-growth phase, underpinned by a sharp rise in emergency and trauma-care demands. According to recent data, the market is set to expand significantly across key geographies including Asia-Pacific, Europe, the United States and Saudi Arabia. In particular, rising incidence of critical vascular access failures in trauma and shock cases is driving rapid adoption of intraosseous devices.
Explore trends before investing – request a sample report today!
(https://www.futuremarketinsights.com/reports/sample/rep-gb-1516)
APAC Emerges as a Fast-Growing Hub
Asia-Pacific (APAC) is showing strong momentum, with regional healthcare infrastructure upgrades and expanding pre-hospital emergency systems accelerating uptake. Data indicate that APAC is projected to be the fastest-growing region in the intraosseous devices domain. For example, Europe’s market was valued at USD 261.9 million in 2023 and is expected to grow at a 6.5 % CAGR to 2030. With countries like India and China seeing rapid urbanisation and rising trauma incidents, APAC presents a robust opportunity for device manufacturers and healthcare service providers alike. This growth highlights the urgency for investment in regional supply chains and emergency-care training models.
Europe and USA Holding Steady with Innovation Focus
In Europe, the intraosseous devices market is expanding steadily with a well-developed emergency-care infrastructure and growing clinical adoption of IO access guidelines. Meanwhile, the United States remains a dominant market driven by advanced EMS protocols, high trauma incidence and rapid deployment of powered and automatic IO systems. While growth rates are comparatively moderate, the USA continues to lead in volume, product innovation and regulatory advancement – making it a reliable base for major industry players. The combination of innovation, regulatory clarity and high adoption of emergency care tools makes the USA and Europe stable backbones for this market’s growth.
Saudi Arabia: GCC Region’s Emerging Advancer
In the Middle East & Africa segment, Saudi Arabia is emerging as a key growth territory for intraosseous devices. Research estimates suggest the Saudi IO market generated around USD 9.2 million in 2023 and is forecast to reach USD 12.7 million by 2030 – a CAGR of approximately 4.7 %. This presents an interesting niche growth region where investment in trauma care infrastructure, military procurement and hospital emergency-access devices is gaining urgency. Device companies targeting the GCC and broader MEA region will find Saudi Arabia a strategic pilot market for wider regional roll-outs.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates
(https://www.futuremarketinsights.com/reports/brochure/rep-gb-1516)
Technology & End-User Trends Driving Market Uptake
Automatic and powered-drill intraosseous devices are gaining elevated interest thanks to faster deployment, lower operator-dependence and enhanced safety features. Research shows that while manual IO needles remain widely used (especially in constrained settings), the automatic segment is leading in innovation and adoption. Hospitals continue to dominate as the primary end-user group, given their high volume of trauma, cardiac arrest and emergency intervention cases. Growth in pediatric-specific IO devices, pre-hospital EMS upgrades and military trauma-care procurement are also key growth themes.
Challenges & Strategic Considerations
Despite the strong growth profile, the market faces certain restraints. In some regions, adoption is slowed by training requirements, procedural risks and cost-pressure in emergency-care budgets. Device manufacturers and healthcare operators alike must invest in training, quality assurance and post-market outcomes to mitigate complications. Additionally, the wide variation in regional reimbursement, regulatory pathways and healthcare maturity creates complexity for global deployment.
Outlook and Strategic Call to Action
With the global intraosseous devices market expanding rapidly and regional growth diversified across APAC, Europe, USA and Saudi Arabia, stakeholders—from device makers to hospital operators and EMS systems—must position themselves proactively.
Buy Report Now – Click Here to Purchase the Report:
(https://www.futuremarketinsights.com/checkout/1516)
Latest Pharmaceuticals Reports:-
Long-acting PEG-rhG-CSF Market
https://www.futuremarketinsights.com/reports/long-acting-peg-rhg-csf-market
Radiopharmaceutical Dispensing System Market
https://www.futuremarketinsights.com/reports/radiopharmaceutical-dispensing-system-market
Caprolactam Market
https://www.futuremarketinsights.com/reports/caprolactam-market
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:-
https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.