Venous Thromboembolism Treatment Market to Reach USD 2.6B by 2035, Driven by Growth in USA, Europe, APAC & Saudi Arabia
The global venous thromboembolism treatment market will rise from USD 1.8B in 2025 to USD 2.6B by 2035, driven by aging populations and minimally invasive.
SPAIN, November 10, 2025 /EINPresswire.com/ -- The global venous thromboembolism (VTE) treatment market is forecasted to grow from USD 1.8 billion in 2025 to USD 2.6 billion by 2035, marking a CAGR of 3.9% over the assessment period. The industry is expected to generate an absolute dollar opportunity of USD 800 million during the decade, supported by rising incidence of VTE among aging populations, increasing hospital procedural volumes, and expanding adoption of minimally invasive vascular intervention technologies across major healthcare regions.
Venous thromboembolism, encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), remains one of the leading preventable causes of morbidity and mortality in hospital settings. With global care providers placing heightened emphasis on early intervention, rapid clot removal, and patient safety, demand for clinically validated device-assisted VTE management continues to accelerate.
Explore trends before investing — request a sample report today!:- https://www.futuremarketinsights.com/reports/sample/rep-gb-1341
In 2025, retrievable inferior vena cava (IVC) filters represent the leading product segment with approximately 52% of market share. The growing preference for retrievable filters is attributed to lower long-term complication risks, improved safety profiles, and their suitability for temporary protection against pulmonary embolism. Manufacturers are investing in improved retrieval mechanisms, enhanced biocompatibility, and device designs optimized for complex patient anatomies.
Hospitals remain the primary end-user category, accounting for roughly 43% of VTE treatment procedures in 2025. This dominance reflects the need for multidisciplinary care coordination, acute care infrastructure, interventional cardiology and vascular surgery capabilities, and emergency-based procedural environments. Ambulatory surgical centers and catheterization laboratories are emerging as secondary care nodes, supporting the trend toward minimally invasive interventions.
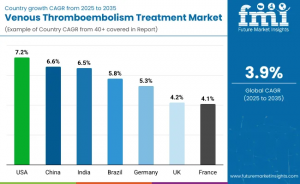
Regional Growth Trends
North America currently leads global VTE treatment adoption, supported by advanced healthcare infrastructure, high awareness of VTE risks, and extensive reimbursement coverage. The United States is projected to post a strong CAGR of 7.2% between 2025 and 2035, driven by rising interventional procedural volumes and ongoing clinical adoption of next-generation thrombectomy devices.
Europe continues to demonstrate mature but stable growth, with Germany, France, and the United Kingdom advancing clinical guideline-driven treatment adoption. These markets benefit from comprehensive insurance coverage frameworks, strong evidence-based practice standards, and established vascular intervention networks.
Across the Asia-Pacific region, China and India are exhibiting accelerated growth at 6.6% and 6.5% CAGR respectively, fueled by rapid modernization of healthcare infrastructure, expanding medical training programs, and growing disease prevalence linked to aging, cancer incidence, and lifestyle factors. Increasing investment in tertiary care hospitals and interventional capabilities is driving both hospital-based and preventative patient management pathways.
Saudi Arabia and the broader GCC are also emerging as important growth regions, supported by government-backed healthcare modernization initiatives and investments in advanced cardiovascular and vascular intervention facilities. Increasing screening efforts and procedural capacity expansion are expected to boost VTE treatment adoption rates in specialized care centers.
Technology and Market Drivers
Market advancement is particularly influenced by clinical transition toward device-enabled clot management. Mechanical thrombectomy systems, catheter-directed thrombolysis (CDT), and hybrid interventional techniques have demonstrated improved clot resolution rates and lower complication profiles when compared to anticoagulation-only treatment models. Clinical studies report thrombectomy achieving up to 85% clot removal efficacy, compared to 45–60% under conventional anticoagulation approaches.
Growing awareness of VTE as a critical patient safety priority, expansion of hospital quality initiatives, and emphasis on early diagnosis are also contributing to rising adoption. Meanwhile, integration of imaging-guided interventional systems and hospital workflow standardization are enhancing procedural efficiency.
However, market growth is constrained by regulatory approval timelines, specialized clinician training requirements, and cost-accessibility barriers in resource-sensitive regions. High unit costs associated with advanced thrombectomy systems and procedural equipment present challenges for widespread adoption outside high-income medical centers.
Click Here to Purchase the Report:- https://www.futuremarketinsights.com/checkout/1341
Competitive Landscape
The market remains moderately consolidated, with leading companies such as Boston Scientific Corporation, Stryker Corporation, AngioDynamics, Cardinal Health Inc., Janssen Pharmaceuticals Inc., and Argon Medical Devices driving product innovation and strategic expansion. Competitive advantage is increasingly tied to clinical outcome validation, device safety performance, and hospital partnership models.
Future Outlook
With global aging trends accelerating and clinical practice shifting toward minimally invasive, outcomes-focused VTE management, the venous thromboembolism treatment market is positioned for steady expansion through 2035. Increased emphasis on patient recovery timelines, reduced procedural risks, and scalable interventional solutions will remain central to market progression.
Latest Therapy Area Reports:-
Medical Eye Shield Film Market
https://www.futuremarketinsights.com/reports/medical-eye-shield-film-market
Medical Far Infrared Therapy Device Market
https://www.futuremarketinsights.com/reports/medical-far-infrared-therapy-device-market
Kids Splint Market
https://www.futuremarketinsights.com/reports/kids-splint-market
Why FMI:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.